Homeopathy & New Zealand legislation
Therapeutic Products Act Repeal Bill 67-1(2024) - my submission
Therapeutic Products Act Repeal Bill
The bill would repeal the Therapeutic Products Act 2023. The effect of this repeal would be that the Medicines Act 1981 and the Dietary Supplements Regulations 1985 would continue in force.
New Zealander’s are encouraged to make their opinions known
The closing date for submissions is 11.59pm on Monday, 29 July 2024
https://www.parliament.nz/en/pb/sc/make-a-submission/
Friday 26 July 2024.
Thank you to Chair Uffindell and Members of the Health Committee for this opportunity to state my individual opinion (with supporting evidence) as a professional homeopath regarding the Therapeutic Products Act Repeal Bill 67-1 (2024) to repeal the Therapeutic Products Act 2023 (the TPA).
Please note that I, Sarah Penrose a New Zealand registered homeopath, have collaborated with other professional homeopaths and support these submissions particularly those made by Lora Hagemann, MAIK(NZ), RCHom(NZ), PhD(USA), BS(USA), and the New Zealand Council of Homeopaths and New Zealand Homeopathic Society joint submission.
As the prior (Labour) Health Select Committee was formulating the TPA, I submitted my individual opinion1 as a New Zealand registered homeopath, outlining my opposition and here, again, for the benefit of the current Health Committee, I cite New Zealand’s critical lack of contemporaneous, comprehensive, and nationally representative data on complementary and alternative medicine (CAM) use;2
and highlight the 2021 Complementary and alternative medicine - practice, attitudes, and knowledge among healthcare professionals in New Zealand: an integrative review which found 80% of GPs referring their patients to CAM practitioners and 25% of GPs themselves practicing CAM – and declared itself the first step to building an insightful evidence base to further the development of effective safety, efficacy, regulatory and integrative CAM policies in New Zealand.3
I now make this submission to the Health Committee in support of the current Therapeutic Products Act Repeal Bill 67-1 (2024) to repeal the TPA, with the inclusion of additional salient facts pertaining to the worldwide implementation and usage of CAM, especially of homeopathy.
An Individual Submission to the Therapeutic Products Act Repeal Bill 67-1 (2024) repealing the Therapeutic Products Act 2023 (the TPA).
Homeopathy is included within the national health systems of a number of countries including Brazil, Chile, India, Mexico, Pakistan, and Switzerland.4
In Switzerland, several complementary and alternative medicine (CAM) options including homeopathy when provided by a certified physician are covered under the mandatory basic health insurance, treatments by non-medical certified CAM providers are partially covered by supplemental optional health insurance.5
India provides an excellent example of the integration of CAM within primary care. The Government Ministry of Ayurveda, Yoga, and Naturopathy, Unani, Siddha and Homoeopathy (Ayush) was formed in 2014 with a vision of ‘reviving the profound knowledge of our ancient systems of medicine and ensuring the optimal development and propagation of the Ayush systems of healthcare.’ (The Department of Indian System of Medicine and Homoeopathy was responsible for the development of Ayush systems from 1995 – 2014).6
During 2022, the Ministry of Ayush in conjunction with the World Health Organization established the first Global Traditional Medicine Centre in Jamnagar, India.7
The first WHO Traditional Medicine Global Summit titled Towards health and well-being for all held in India during 2023 included G20 government officials and ‘looked anew at the application of rigorous scientific methods to unlock the vast potential of traditional, complementary and integrative medicine (TCIM) amidst important challenges and opportunities to realize universal health coverage and promote health and well-being for people and the planet’8 with the Gujarat Declaration as outcome document.
‘The 2023 Gujarat Declaration recognizes that billions of people use Indigenous knowledges, resources and methods, and TCIM, for their health and well-being, and that for many it is their only or preferred option for health care.’9
The Gujarat participants ‘reaffirmed global commitments related to indigenous knowledges, biodiversity and TCIM’ including the:
· Declaration of Alma-Ata of 1978
· Convention on Biological Diversity 1992
· UN Declaration on the Rights of Indigenous Peoples 2007
· UN 2030 Agenda for Sustainable Development
· Astana Declaration on primary health care 2018
· UN General Assembly political declaration on universal health coverage in 2019
· World Health Assembly resolutions on TCIM, and Indigenous People’s health and rights, among others.10
By contrast, New Zealand’s Wellbeing Budget of 2024 announced ‘a substantial contribution to the healthcare sector to deliver high quality healthcare to New Zealanders’11 with no mention of TCIM / CAM. (NB. implemented in 2019, Wellbeing Budgets aim at gauging the long-term impact of policies on the quality of people’s lives.)
In fact, the last time TCIM / CAM was mentioned within a New Zealand budget was in 2008 under ‘A Framework for Integrative Primary Care’ suggesting this was a program of work to develop an evidence-based framework for sector wide agreement regarding the outlining of the principles and models for integrating TCIM/CAM into mainstream primary health care.12 (NB. the commissioned report for this work is not publicly available on the Ministry of Health website, and therefore, we have no transparency on how far this work progressed and why it was dropped.)

Regarding the current New Zealand Government’s intended modernised regime for natural health products13 it must be entered into record and made clear to the Health Committee that the widely quoted Australian National Health and Medical Research Council (NHMRC) report on homeopathy14 is not scientifically reliable to inform decision making regarding the efficacy of homeopathy.
The NHMRC report on homeopathy (2015) concluded that “…there are no health conditions for which there is reliable evidence that homeopathy is effective”15 triggering worldwide headlines that homeopathy does not work for any condition. However, in 2019 Professor Anne Kelso (the then NHMRC CEO) stated that “Contrary to some claims, the review did not conclude that homeopathy was ineffective.”16
The NHMRC Australian Review stakeholder complaint17 resulted in a seven-year long Ombudsman investigation to address scientific misconduct within the NHMRC report on homeopathy (2015) highlighted the NHMRC’s use of unprecedented and flawed scientific methods including:18
· The NHMRC conducted a homeopathy review twice producing two reports in July 2012 and March 2015.
· The existence of the first (July 2012) report was hidden from the taxpayers who funded it – and was discovered through Freedom of Information (FOI) requests.
· The NHMRC rejected the first (July 2012) report due to poor quality despite it being undertaken by a reputable scientist and author of NHMRC’s internal guidelines of conducting evidence reviews.
· The NHMRC stated the results of the second (March 2015) report were based on a “rigorous assessment of over 1800 studies” when in fact based on just 176 studies.
· FOI requests revealed Professor Fred Mendelsohn an NHMRC expert committee member overseeing the review process confirmed the first (July 2012) review to be of high quality stating “I am impressed by the rigor, thoroughness and systematic approach given to this evaluation [….] Overall, a lot of excellent work has gone into this review and the results are presented in a systematic, unbiased and convincing manner.”19
· The NHMRC implemented an arbitrary method (not used elsewhere) that trials had to have at least 150 participants and reach an unusually high threshold for quality to be deemed reliable despite the fact that the NHMRC regularly conducts studies with less than 150 participants.20
· The unprecedented and arbitrary NHMRC rules classified 171 trials as unreliable leaving just 5 which were all assessed as negative trials resulting in the NHMRC conclusion of no ‘reliable’ evidence for homeopathy.21
· The signed conflict of interest form of Professor Peter Brooks NHMRC committee Chair of the second (March 2015) report declared he was not “affiliated or associated with any organisation whose interests are either aligned with or opposed to homeopathy” despite being a member of anti-homeopathy lobby group Friends of Science in Medicine.
· The NHMRC guidelines state that topic experts must be included yet there was no homeopathy expert on the second (March 2015) report committee.
In August 2023, the Australian Ombudsman’s Office closed the seven-year NHMRC scientific misconduct complaint stating:
“Despite our best efforts, it was not possible to engage an expert (or experts) to provide independent advice to our Office on this subject. In the absence of independent, expert scientific expertise we have not been able to conclusively determine those matters of scientific methodology.”22
The current Australian Natural Therapies Review 2019-2023 submitted evidence document contains over 130 pages of homeopathy studies.24
The current state of the available evidence for homeopathy
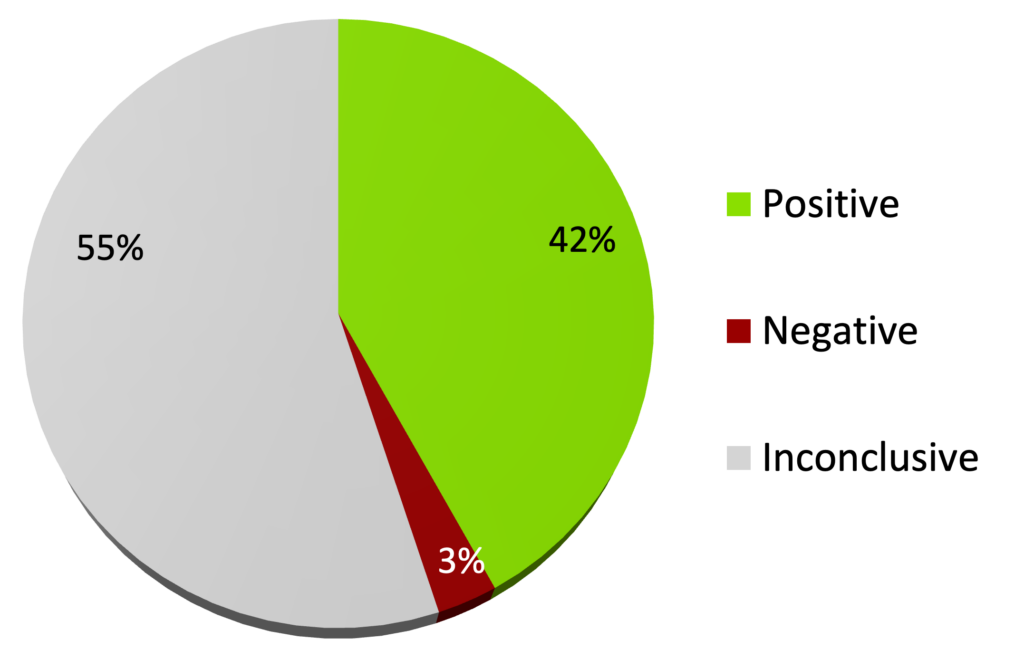
By the end of 2023, 166 double blind randomized controlled trials (DB-RCTs) of homeopathy in one hundred medical conditions had been published in peer reviewed journals.25
166 DB-RCTs of homeopathy (end of 2023)
Cochrane review of systematic reviews of predominantly conventional therapies (2007) showed 43% of conventional therapies are effective.26 A systematic review (2022) stated that homeopathy should be provisionally classified within the group of conventional therapies which are effective but need further research27 based upon the results of 2014,28 2017,29 and 201830 meta-analyses.
The researchers of the 2023 Efficacy of homoeopathic treatment: Systematic review of meta-analyses of randomised placebo-controlled homoeopathy trials for any indication31 stated:
‘In contrast to frequent claims, the available MAs of homoeopathy in placebo-controlled randomised trials for any indication show significant positive effects beyond placebo. Compared to other medical interventions, the quality of evidence for efficacy of homoeopathy was similar or higher than for 90% of interventions across medicine.32 Accordingly, the efficacy evidence from placebo-controlled randomised trials provides no justification for regulatory or political actions against homoeopathy in health-care systems.’
In conclusion, this submission supports the Therapeutic Products Act Repeal Bill 67-1 (2024) repealing the Therapeutic Products Act 2023 (the TPA) and introduces robust current evidence for homeopathy which I respectfully urge the Chair and Members of the Health Committee to ensure is included for consideration within the consultation and construction processes of the proposed modernised regime for natural health products intended to replace the TPA. If required, I am available to the Chair and Members of the Health Committee to assist with any homeopathy-based enquiry.
Sincerely,
Sarah Penrose
Bachelor of Science (Honours) homeopathy (UK 2021)
Diploma homeopathy (Greece 2022; New Zealand 2010)
New Zealand Registered Homeopath
Member of the New Zealand Homeopathic Society
General member of the Australian Homeopathic Association
Associate member of Liga Medicorum Homeopathica Internationalis
New Zealander’s are encouraged to make a submission to the Therapeutic Products Act Repeal Bill 67-1(2024)
The closing date for submissions is 11.59pm on Monday, 29 July 2024
https://www.parliament.nz/en/pb/sc/make-a-submission/
Homeopathy & NZ's Therapeutic Products Bill
The latest out of New Zealand is the imminent Therapeutic Products Bill intended to replace the Medicines Act 1981 and the Dietary Supplements Regulations 1985 to provide for the comprehensive, risk-proportionate regulation of therapeutic products, such as medicines, medical devices, natural health products, and active pharmaceutical ingredients.
Lee et al., 2021. Mapping prevalence and patterns of use of, and expenditure on, traditional, complementary and alternative medicine in New Zealand: a scoping review of qualitative and quantitative studies. N Z Med J. Sep3;134(1541):57-74. Available from: https://pubmed.ncbi.nlm.nih.gov/34531597
Liu et al., 2021. Complementary and alternative medicine - practice, attitudes, and knowledge among healthcare professionals in New Zealand: an integrative review. BMC complementary medicine and therapies. 21(1), 63. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7882070/
Homeopathy Research Institute, 2024. Homeopathy use around the world. Available from: https://www.hri-research.org/resources/essentialevidence/use-of-homeopathy-across-the-world/
Klein SD, Torchetti L, Frei-Erb M, Wolf U. Usage of Complementary Medicine in Switzerland: Results of the Swiss Health Survey 2012 and Development Since 2007. PLoS One. 2015 Oct 29;10(10):e0141985. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4626041/
Government of India. 2024. Ministry of AYUSH. Available from: https://ayush.gov.in/
Government of India. 2024. Ministry of AYUSH. A Decade of Transformative Growth in Ayush. Towards Holistic Health for All. 2014-2024. Collaborations with WHO (Pp55). Available from: https://ayush.gov.in/images/annualReport/DecadeAyushReport.pdf
World Health Organisation, 2023. WHO Traditional Medicine Global Summit 2023 meeting report: Gujarat Declaration. Available from: https://www.who.int/publications/m/item/who-traditional-medicine-summit-2023-meeting-report--gujarat-declaration
World Health Organisation, 2023. WHO Traditional Medicine Global Summit Towards health and well-being for all Gandhinagar, Gujarat, India, 17-18 August 2023. https://www.who.int/news/item/21-03-2024-charting-an-evidence-based-roadmap-for-who-global-traditional-medicine-centre-collaborations
Ibid.
WilliamBuck. New Zealand Budget Update 2024. https://williambuck.com/nz/tools/nz-budget-update-2024/#1589504204156-3af65c92-b563
Te Tai Ohanga The Treasury, 2024. Information supporting the estimates of appropriations Health Sector - Information Supporting the Estimates of Appropriations for the Government of New Zealand for the Year Ending 30 June 2009 - Performance Information for Appropriations Vote Health B.5A Vol.6 (Pp43) Available from: https://www.treasury.govt.nz/sites/default/files/2008-05/ise08-v6-pia-health.pdf
Beehive.govt.nz, 2024. Therapeutic Products Act to be repealed. Hon. Casey Costello. 08 May. Available from: https://www.beehive.govt.nz/release/therapeutic-products-act-be-repealed
Australian Government. National Health and Medical Research Council. 2023. Homeopathy. Available from: https://www.nhmrc.gov.au/about-us/resources/homeopathy
Effectiveness of Homeopathy for Clinical Conditions: Evaluation of the Evidence. Overview Report. Prepared for the NHMRC Homeopathy Working Committee by Optum. October 2013. Available from: https://www.hri-research.org/wp-content/uploads/2014/07/Homeopathy-Overview-Report.pdf
Australian Government. National Health and Medical Research Council. 2019. CEO Statement Release of an annotated version of the 2012 draft report The Effectiveness of Homeopathy: an overview review of secondary evidence. Available from: https://www.nhmrc.gov.au/sites/default/files/documents/attachments/CEO-statement-signed.pdf
The National Health & Medical Research Council (NHMRC) and Research Integrity, Australian Homeopathic Association. 2017. Executive Summary - Commonwealth Ombudsman Complaint. Available from http://www.nhmrchomeopathy.com/ombudsman-exec-summary.html
Homeopathy Research Institute, 2024. The Australian Report. Available from: https://www.hri-research.org/resources/homeopathy-the-debate/the-australian-report-on-homeopathy/
Homeopathy Group. 2019. Release The First Report. Homeopathy: No better than placebo? Available from: https://releasethefirstreport.com/the-full-story
Homeopathy Research Institute, 2024. The Australian Report FAQ’s. Available from: https://www.hri-research.org/resources/homeopathy-the-debate/the-australian-report-on-homeopathy/australian-report-faqs/
Ibid.
Commonwealth Ombudsman, 2023. Finalisation of investigation relating to the National Health and Medical Research Council's review of the evidence for the effectiveness of homeopathy. Available from: https://www.ombudsman.gov.au/__data/assets/pdf_file/0008/300014/NHMRC-2023-Statement.pdf
Australian Government Department of Health and Aged Care. The Australian Natural Therapies Review 2019-20. Available from: https://www.health.gov.au/topics/private-health-insurance/private-health-insurance-reforms/natural-therapies-review-2019-20
Australian Government Department of Health and Aged Care. The Australian Natural Therapies Review 2019-20. Tranche 2 submitted evidence. Available from: https://www.health.gov.au/sites/default/files/2024-04/tranche-2-submitted-evidence.pdf
Homeopathy research Institute, 2024. What scientific evidence is there that homeopathy works? Available from: https://www.hri-research.org/resources/homeopathy-faqs/scientific-evidence-for-homeopathy/
El Dib et al., 2007. Mapping the Cochrane evidence for decision making in health care. J Eval Clin Pract. Aug;13(4):689-92. Available from: https://pubmed.ncbi.nlm.nih.gov/17683315/
Weiermayer et al., 2022. Evidence-Based Human Homeopathy and Veterinary Homeopathy. Comment on Bergh et al. A Systematic Review of Complementary and Alternative Veterinary Medicine: "Miscellaneous Therapies". Animals (Basel). Aug 17;12(16):2097.Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9404715/
Mathie et al., 2014. Randomised placebo-controlled trials of individualised homeopathic treatment: systematic review and meta-analysis. Syst Rev. Dec 6;3:142. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4326322/
Mathie et al., 2017. Randomised, double-blind, placebo-controlled trials of non-individualised homeopathic treatment: systematic review and meta-analysis. Syst Rev. Mar 24;6(1):63. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5366148/
Mathie et al., 2018. Systematic Review and Meta-Analysis of Randomised, Other-than-Placebo Controlled, Trials of Individualised Homeopathic Treatment. Homeopathy. Nov;107(4):229-243. Available from: https://pubmed.ncbi.nlm.nih.gov/30121049/
Hamre et al., 2023. Efficacy of homoeopathic treatment: Systematic review of meta-analyses of randomised placebo-controlled homoeopathy trials for any indication. Systematic Rev. Oct 7;12(1):191. https://systematicreviewsjournal.biomedcentral.com/articles/10.1186/s13643-023-02313-2#Abs1
Howick et al., 2022. Most healthcare interventions tested in Cochrane Reviews are not effective according to high quality evidence: a systematic review and meta-analysis. J Clin Epidemiol. Aug;148:160-169. Available from: https://pubmed.ncbi.nlm.nih.gov/35447356/





Sarah- Thank you for sharing this full article on homeopathy and where it stands in the governmental chain. Although as one with a deep interest in places, the thing that got stuck in my head is the inevitable Beehive. 😁
"Homeopathy is included within the national health systems of a number of countries including Brazil, Chile, India, Mexico, Pakistan, and Switzerland.4"
You can add Iran to the list.
I once had the honour of speaking with Dr Ardavan Shahrdar who was head of the Faculty of Homeopathy, within the Ministry of Health of Iran. He said that due to economic sanctions by the USA, Iran did not have access to many pharmaceutical drugs and Homeopathy was used extensively instead. He mentioned that often his medical training got in the way of his homeopathic prescribing. He was a great teacher but unfortunately Iran blocked access to www so we can no longer benefit from his knowledge.